Share Important Moment of MileCell Bio with You
2025.11.29
Peripheral blood mononuclear cell (PBMC) cryopreservation is essential for biobanking, clinical trials, and immune monitoring studies. However, unoptimized freezing/thawing protocols inflict multifaceted damage, compromising cellular integrity and functionality. Post-thaw viability loss-routinely exceeding 20–30% in suboptimal conditions-stems primarily from ice crystal-induced membrane rupture, osmotic shock, and apoptosis triggered during the freeze-thaw continuum.
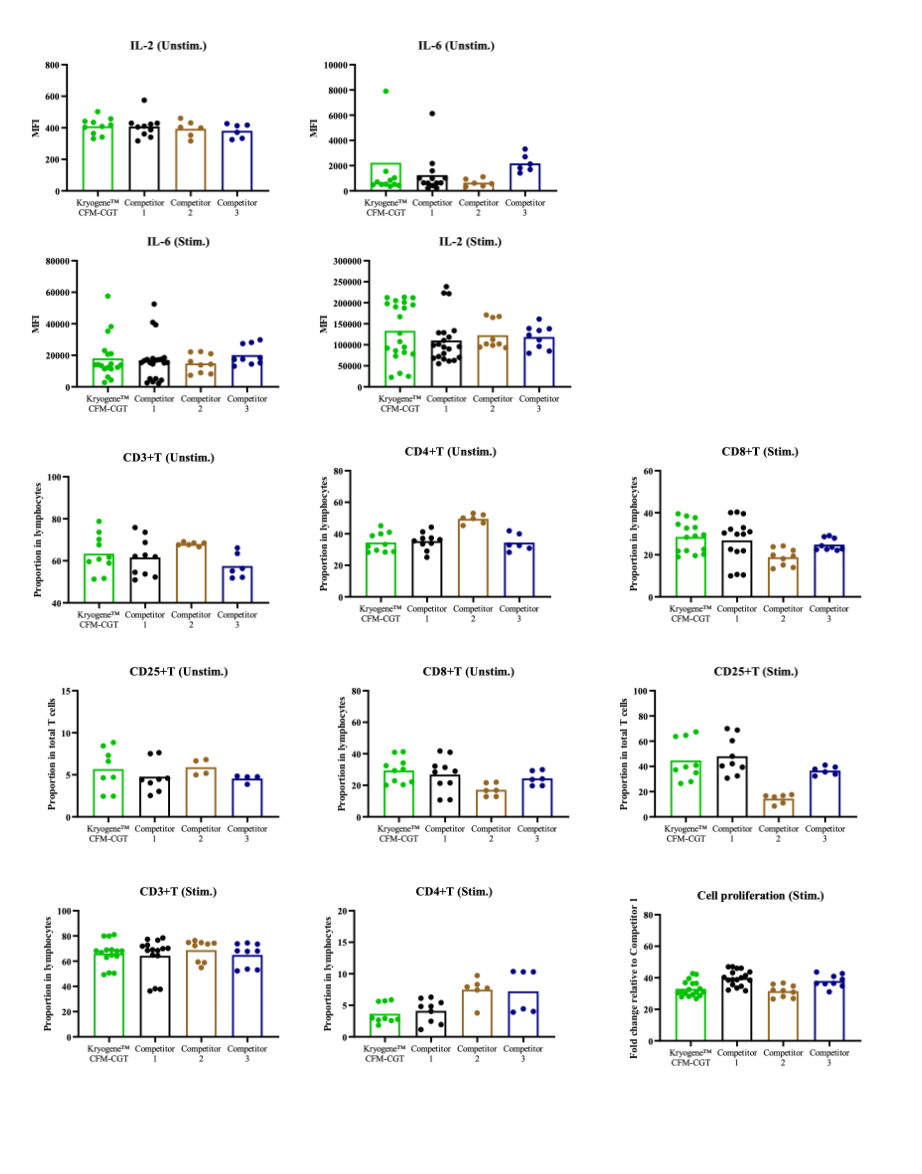
Beyond viability, cryopreservation alters immunophenotypic profiles. Key markers such as CD62L (L-selectin) and CCR7 exhibit significant down-modulation, impairing lymphocyte homing capacity. T-cell subsets suffer disproportionate attrition: CD8+ effector memory (TEMRA) and regulatory T cells (Tregs) show heightened susceptibility to freeze-thaw stress compared to naive counterparts.
Functional impairments are equally consequential. Thawed T cells demonstrate attenuated cytokine secretion profiles-notably reduced IFN-γ, TNF-α, and IL-2 production-upon polyclonal (anti-CD3/CD28) or antigen-specific stimulation. This hyporesponsiveness correlates with disrupted calcium flux and TCR signaling cascade dysregulation.
Compounding this deficit, cryopreservation erodes proliferative capacity: CFSE dilution assays reveal up to 30% reduction in mitotic potential post-thaw, attributed to DNA fragmentation and metabolic quiescence.
These impairments not only diminish experimental sensitivity but may bias clinical correlates in longitudinal studies. Consequently, meticulous validation of cryopreservation workflows-spanning freezing media, cooling kinetics, storage conditions, and thaw protocols-is nonnegotiable for preserving immune cell functionality across research and translational applications.
PBMCs from multiple donor sources were cryopreserved using Kryogene™ Cell Freezing Media-CGT, as well as competitor 1, competitor 2, and competitor 3. After thawing, cell viability and function were assessed. PBMCs were stimulated with PMA-Inomycin for activation, and cytokine release was measured using the CBA method. T-cell activation and phenotypes were analyzed via flow cytometry. PBMCs labeled with Celltrace were stimulated with CD3/28 beads to assess cell proliferative activity using flow cytometry.